
Soluble in water, aqueous solution is alkaline; insoluble in ethanol, acetone and ether. Strong moisture absorption, carbon dioxide and water can be absorbed when exposed to the air and changed into potassium bicarbonate. Its hydrate includes Monohydrate, dihydrate, trihydrate, tetrahydrate.
Potassium carbonate has anhydrous substances or crystals containing 1.5 molecules. Anhydrous is white granular powder, crystal is white semitransparent small crystal or particle, odorless, strong alkali, relative density of 2.428 (19℃), melting point 891℃, When exposed to air, it will absorb carbon dioxide and moisture, turn into potassium bicarbonate, Hence sealed packaging is required.
Production process:
Ion exchange: exchange of cation exchange resin with potassium chloride. Ammonium bicarbonate was then removed form dilute solution of potassium bicarbonate, which was produced by multi effect evaporation, carbonization, crystallization, separation and calcination.
Applications:
It is mainly used for the analysis of reagents. Such as high purity analysis, emission spectrum analysis and so on. It can also be used as flux for silicate and insoluble sulphate, water absorbent for organic liquid, electroplating, fertilizer and photographic industry.
Used in glass, printing and dyeing, soap, enamel, preparation of potash, synthetic ammonia decarbonylation as well as in color TV industry. it used as puffing agent in food, gas adsorbent, dry powder fire extinguishing agent, rubber antiager, and so on; used for the processing of exposed photosensitive materials.
Used in the manufacture of the glass shell of the electronic industry, the decarburization of chemical fertilizer production and the manufacture of potash salts, used as analytical reagents, fluxes, and also for the preparation of various potash salts; used for the analysis of reagents, reference agents and molten silicate and insoluble sulphate fluxes.
Potassium carbonate structural formula
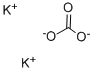
In chemical experiments, it is used as a desiccant. Its ability to absorb water is moderate, and it can form potassium carbonate (K2CO3•2H2O) with two crystal water, but its action with water is slow. It is suitable for drying neutral organic compounds such as alcohols and esters, as well as ordinary alkaline organic compounds such as amines and alkaloids. But it can not be used as a desiccant for acids, phenols or other acidic substances
Attentions:
Complete packing and safe loading is required. Please ensure packing no leak, collapse, fall or damage. It is strictly prohibited to be mixed with oxidizer, acid, food chemicals, etc. Transportation should be protected from exposure, rain, high temperature. The vehicles should be thoroughly cleaned after transportation.